Safeγ UCAR-γδT Platform
Technology Platform 1440 Views 2020-04-08 10:59
The Safeγ UCAR-γδT Platform is an innovative universal cell immunotherapy technology based on CAR-modified Vδ1 T cells. Vδ1 T cells account for less than 1% of total T cells in the periphery and are primarily found in the thymus, mucosal epithelial tissues (e.g., skin, intestine, spleen, and liver), which poses significant challenges for large-scale production of Vδ1 T cells in vitro. The unique cellular properties of Vδ1 T cells endow them with strong anti-tumor potential. PersonGen has been developing UCAR-Vδ1T products since 2018 and has successfully established a unique manufacturing process for the commercial, large-scale production of Vδ1 T cells under cGMP conditions. The company has also addressed challenges related to lentiviral transduction and cryopreservation of UCAR-Vδ1T cells. Currently, PersonGen's UCAR-Vδ1T cell platform has emerged as an international leader in immunotherapy innovation, with its groundbreaking research consistently published in top-tier oncology journals, demonstrating its pioneering role in advancing cancer treatment paradigms.
The platform has the following core features and advantages:
Platform Features:
Unique Advantages of γδT Cells
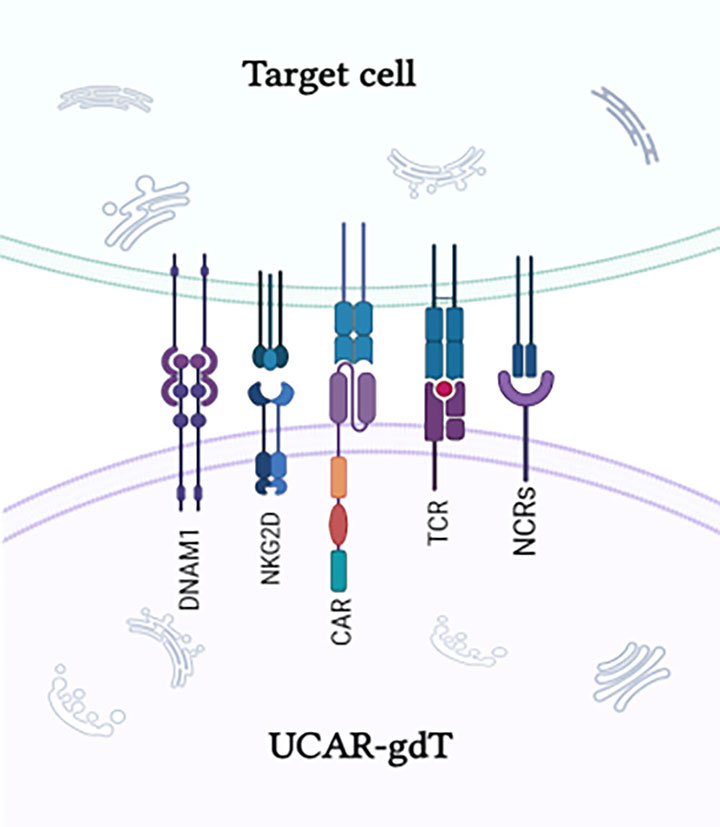
γδT cells are natural surveillance cells of the immune system, combining adaptive and innate immune characteristics. They recognize tumor antigens in a non-MHC-restricted manner, playing a critical role in tumor immunosurveillance, particularly in solid tumor therapy.
Active Homing Ability
γδT cells, especially Vδ1 subtypes, possess strong active homing capabilities, enabling directed migration to tumor tissues like "tracking missiles," accurately targeting and killing tumor cells.
No Gene Editing Required
Unlike traditional CAR-T cell therapies, γδT cells can be developed into universal, off-the-shelf cell therapy products without gene editing, reducing production costs and risks associated with gene editing.
Safety and Efficacy
γδT cell therapies have shown low risks of cytokine release syndrome (CRS) and graft-versus-host disease (GvHD) in clinical trials. Additionally, they demonstrate excellent anti-tumor activity in both solid tumors and hematological malignancies. The Safeγ UCAR-γδT Platform modifies γδT cells into CAR-γδT cells, leveraging their natural advantages to achieve strong specific killing of tumor cells expressing target antigens.
Platform Advantages:
Research Progress
UTAA09 Injection: A universal product targeting B-cell tumors and autoimmune diseases. Preclinical data show excellent in vivo efficacy, and an IND application has been officially submitted.
UTAA06 Injection: A universal CAR-T cell product targeting solid tumors and acute myeloid leukemia (AML). Preclinical data demonstrate high rates of complete remission (CR) in multiple solid tumor mouse models after a single administration, with excellent tumor tissue infiltration capabilities.
Commercialization and Patient Accessibility
The Safeγ UCAR-γδT Platform addresses key technical challenges such as closed-system large-scale production, CAR structure optimization, lentiviral transduction, and cryopreservation formulation development. Compared to other CAR-T cell therapies, it enables allogeneic treatment while significantly reducing production costs and improving patient accessibility, offering a promising solution for affordable cell therapies.
Global Competitiveness
The Safeγ UCAR-γδT Platform has significant global competitiveness, with its unique technical approach positioning it as a leading therapy in the field of universal cell treatments worldwide.
▲The image is sourced from the Internet, and the copyright belongs to the original author. If the intellectual property rights are unintentionally infringed, please contact us to remove it.