CD7-CAR-T platform
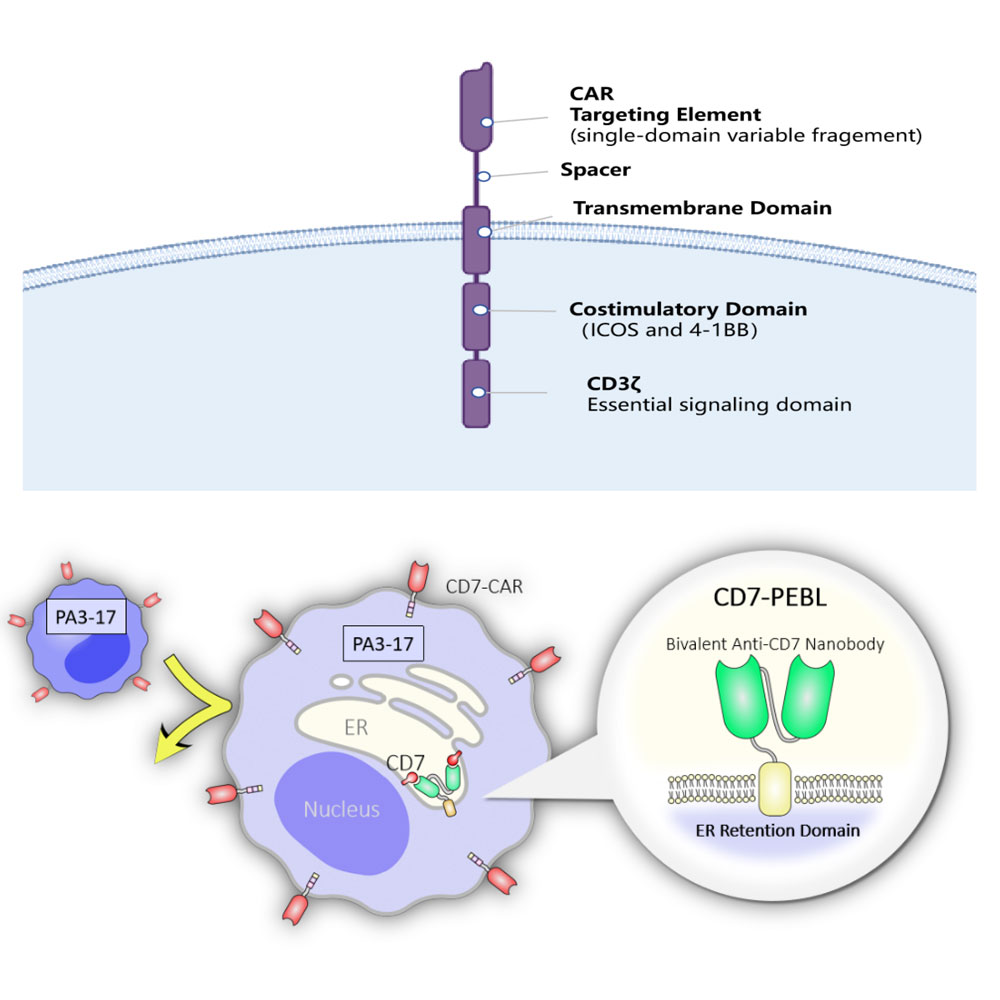
Since normal T cells also express the CD7 molecule, to address the self-destruction phenomenon observed during CD7-CAR-T preparation, researchers pioneered the use of CD7 nanobodies to recombine and express them with an endoplasmic reticulum retention sequence. This strategy confines T cell CD7 antigens within the endoplasmic reticulum and Golgi apparatus, creating a CD7-negative state on T cell surfaces. This approach effectively prevents "self-destruction" among CAR-T cells, ultimately enabling successful production of CD7-CAR-T cells.
The mechanism of CD7-CAR-T cells involves transducing T cells with both CD7-CAR lentivirus and CD7 membrane-blocking lentivirus. This dual-transduction approach creates CD7-CAR-T cells that lack CD7 surface expression, preventing immune cell-mediated "self-destruction" while enabling the cells to specifically recognize and eliminate CD7-positive T-cell tumors through their unique CD7-CAR molecules.
Brightened:
The worlds leading CAR-T product for T-cell malignancies
Ex vivo CAR-T products that cannot be disrupted by in vivo CAR-T