CompanyNews
CompanyNews
Media | CompanyNews 9505 Views 2022-07-05 17:55
Suzhou,China (Jul 5, 2022)- PersonGen Biotherapeutics, a clinical-stage biotech focused on developing transformative cell therapies that are affordable to patients, today announced that the Center for Drug Evaluation (CDE) of NMPA has cleared the company’s Investigational New Drug (IND) application for TAA06, an engineered autologous cell therapy targeting B7-H3 for the treatment of R/R Neuroblastoma.
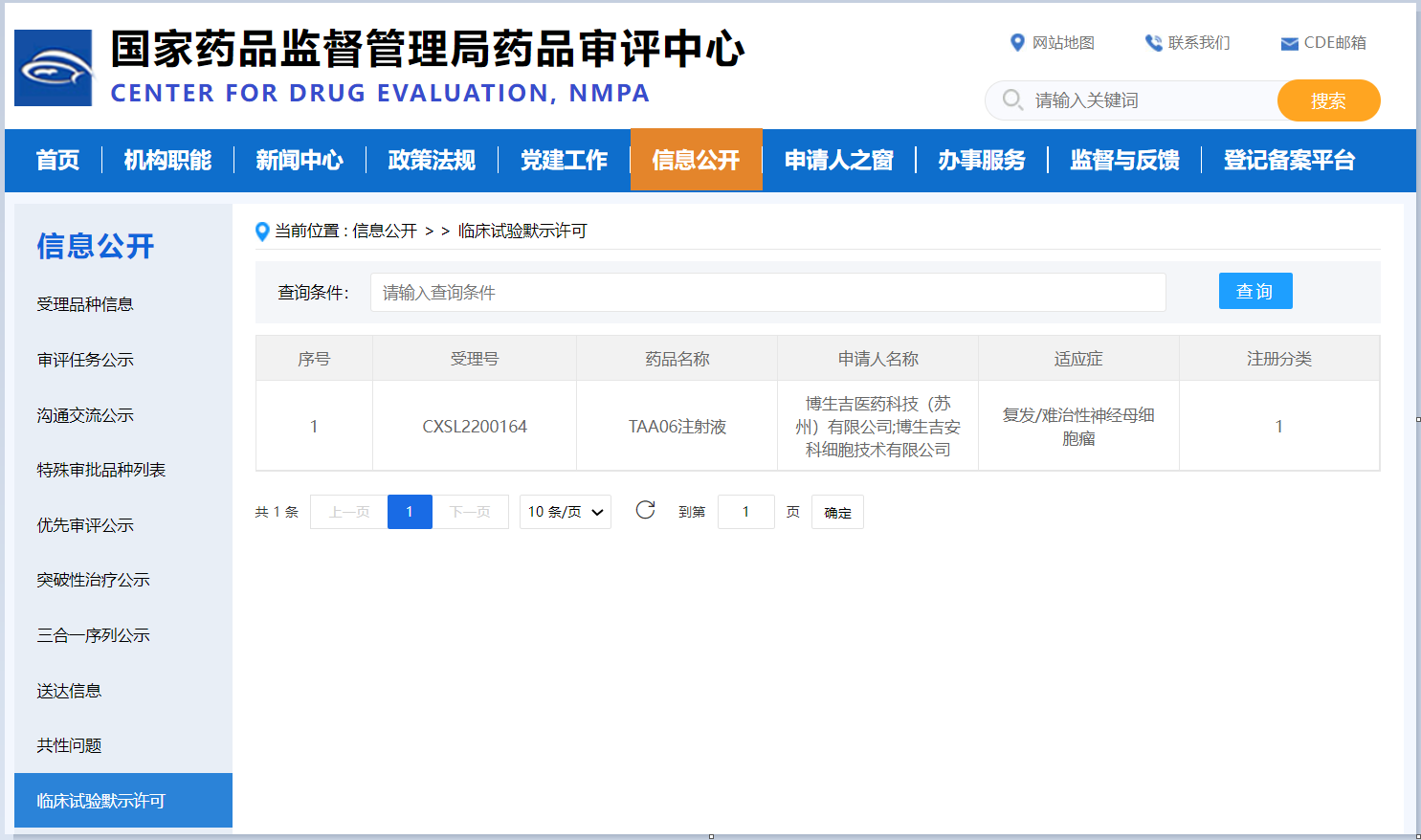
TAA06 injection is PersonGen’s first CAR-T cell therapy for the treatment of solid tumors, and has demonstrated remarkable in vitro/in vivo efficacy data and safety data. In March 2022, PersonGen announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) and Rare Pediatric Disease (RPD) designation to TAA06 for the treatment of R/R Neuroblastoma. The IND approval suggests TAA06 is the first autologous B7-H3-targeted CAR-T cell therapy targeting pediatric solid tumors entering the clinic (registered trial) in China. The clearance of the IND for TAA06 by the NMPA is another significant milestone in the development of cell therapies by PersonGen, and marks the beginning of clinical development of a deep pipeline of CAR-T cell products targeting solid tumors.
About Neuroblastoma
Neuroblastoma is a childhood embryonic tumor of migrating neuroectodermal cell derived from the neural crest and destined for the adrenal medulla and the sympathetic nervous system, a common extracranial solid tumor that occurs almost exclusively in children, and its incidence ranks third in childhoos tumors, second only to leukemia and brain tumor. The incidence of NB is age-related, with an average age of 17.3 months at clinical diagnosis, and 40% of children were diagnosed before 1 year old. About 30% of NB tumors occur in the adrenal medulla, 60% in the abdominal paravertebral ganglia, and the rest in the sympathetic ganglia of the chest, head, neck and pelvis. NB is heterogeneous, and it shows that the survival rate is 85%-90% in low- and intermediate-risk children, while less than 50% in high-risk NB children. High-risk NB children remain refractory after multiple intensive treatments, more than 50% of the children relapse, and the 5-year survival rate is about 40% to 50%. Therefore, it is very necessary to develop effective treatment options for high-risk NB.
About TAA06 Injection
B7- H3 (also known as CD276) is a Type I transmembrane protein that belongs to the B7 immune co-stimulatory and co-suppressive family. It is a promising target with limited expression in normal cells and high expression in malignant solid tumors such as pancreatic cancer, prostate cancer, ovarian cancer, lung cancer, clear cell renal carcinoma, osteosarcoma, Ewing sarcoma, and glioma. In addition to adult solid tumors, B7-H3 is also highly expressed in many pediatric solid tumors (neuroblastoma, Ewing's sarcoma, etc.), which indicates that TAA06 is a promising drug candidate for the treatment of pediatric solid tumors.
About PersonGen
PersonGen's vision is to be a pioneer in the research and development of innovative cell drugs. The mission of PersonGen is to develop innovative drugs to address unmet medical needs. PersonGen is a domestic high-tech enterprise focusing on the development of ground-breaking immunotherapy technology and cellular drug products against oncology. We are dedicated to develop globalal-leading first-in-class and best-in-class CAR-T cell drugs to deliver excellence to cancer patients.
Autologous CD7-CAR T cells, developed by PersonGen, demonstrates high clinical value for the treatment of refractory and recurrent T cell and NK cell malignancies. Its IND application has been approved by CDE in August 2021, and the ODD has been granted by US FDA in November 2021. The B7-H3 targeting CAR-T cell therapy were previously received Orphan Drug Designation (ODD) and RPD (Rare Pediatric Disease) Designation from the United States Food and Drug Administration (FDA) in March 2022 and its IND application has been approved by CDE in July 2022.
In the first half of 2021, PersonGen completed the A round of financing led by Yuansheng Venture Capital, with Puen Guoxin Equity Investment and Xiantong Capital. In January 2022, we completed B round of financing jointly led by Zhongjin Qide and Huatai Zijin, with Panyi Capital and Huatong Capital. PersonGen has built a fully automated CAR-T cell manufacture center of 10,000 square meters in compliance with GMP standards, laying a solid foundation for the commercialization of CAR-T cells.